The idea of nuclear fission
the secrets of uranium | the idea of fission.
This reading is part of a series: Nuclear Waste Disposal
Uranium and its secret:
Nuclear fuel, when it is in ore form, is an unremarkable element naturally found in rock or mineral deposits. It is quite abundant; 40 times more common than silver and 500 times more common than gold [1]. It is found in huge quantities and sufficient concentration in seawater that scientists have explored the economic feasibility of extracting it from the oceans [2].
In its natural form, its radioactivity is benign. But, like many heavy metals, it is chemically toxic. We use substances in daily use that have equal or greater toxicity than uranium, e.g. arsenic and cyanide, which are used for industrial purposes [4].
Something happens to uranium, as its atomic potential is exploited to generate electricity, that it ends up as a highly radioactive and high heat emitting waste when it exits the belly of a nuclear reactor. The “spent fuel” is a million times more radioactive than natural uranium [5]. Its radioactivity is so high that it spontaneously generates its own heat - as much as 2MW/tonne, enough to power 300 homes.
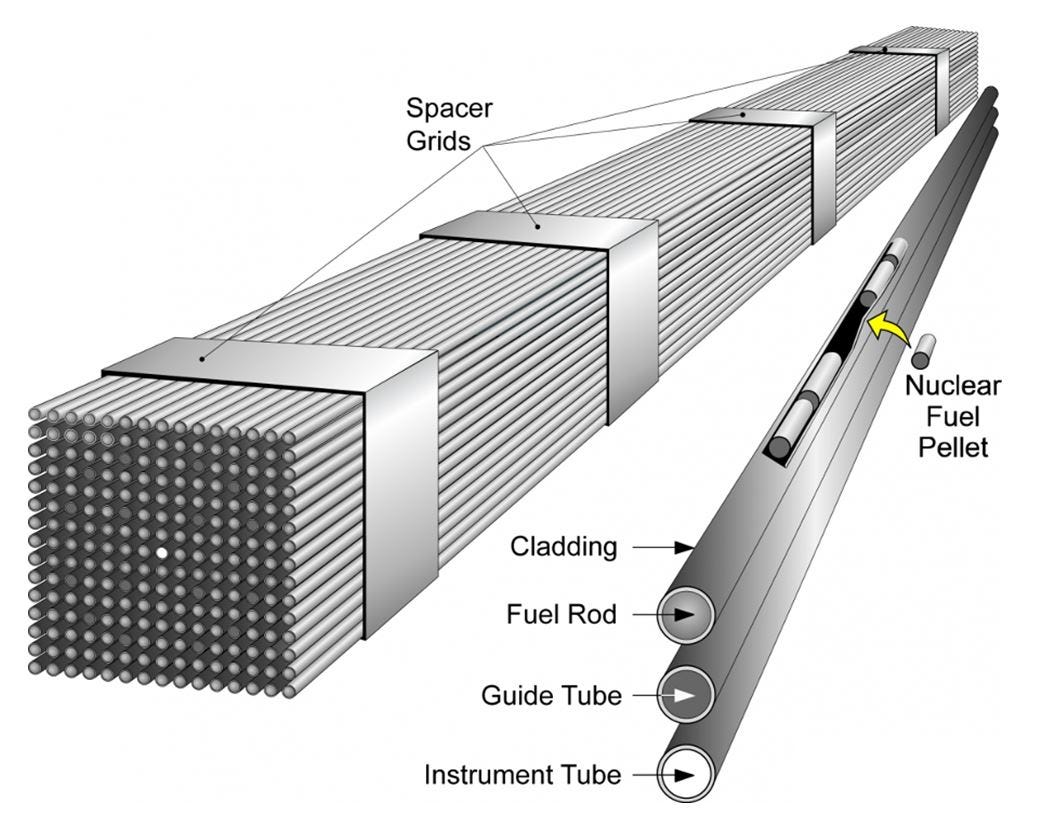
Assembling nuclear fuel:
Natural uranium is mostly U-238 (99.3%) with very tiny amounts of U-235 (0.7%) and U-234 (the other isotopes of uranium. Isotopes of uranium have almost identical chemical properties but slightly different atomic masses). It is U-235 that fissions in a nuclear reactor, and most modern reactors require a U-235 concentration of 3-5%. To achieve this, the fuel is enriched using various methods. In the process, 8 tonnes of natural uranium gives rise to 1 tonne of enriched uranium [6].
The enriched form of uranium is converted to a powder of uranium dioxide which is compacted into cylindrical pellets the size of a pencil eraser. One such pellet contains approximately the same amount of energy as 800 kg of coal [7]
The pellets have a ceramic, porcelain-like structure. These pellets are stacked and sealed in metal tubes made of zirconium alloy (for their low neutron absorption) that are 12 feet long and ½ inch in diameter [8] to form fuel rods. The metal tubes hold the fuel and any gases released during fission.
Fifty of these fuel rods are bound together to form a fuel assembly. A modern reactor core can contain 200 of these fuel assemblies with a total of 110 tons of uranium fuel [8]. Fuel assemblies are carefully designed to allow water to flow through them, taking away the heat generated by fission.
Nuclear fuel has an active life of around three years, during which most of the energy output comes from the fission of U-235. The reactor also converts some of the U-238 in the fuel into plutonium (Pu-239) by neutron capture, and half of that plutonium also fissions and contributes to energy production, providing about one-third of the reactor’s energy output over this time [9].
After spending about three years in a reactor, the fuel transforms into high-level radioactive waste. This “spent fuel” still has about 1.0% U-235 and 0.6% fissile plutonium, with around 95% U-238. The balance, about 3%, is fission products and minor actinides [9].
The basic idea of Nuclear Fission:
How does nuclear fuel release energy? Imagine a restaurant that is oddly named ‘the Nuclear Reactor.’ Here, the waiters, all wearing a tag confusingly saying ‘U-235’, go about so laden with dishes that they are almost at their limit. They are trying to balance the numerous plates starting from the first they lift with their fingers, the next they rest on their palm and the next on their forearm, with plates stacked, one on top of another. It is quite a balancing act as they navigate the crowded restaurant. And as they move around, they wobble, and the plates rattle, and one can see the entire thing about to collapse at any instance. But somehow, they know exactly how much to carry and do their job well. They are always on the verge of crashing but never do.
The new chef that the restaurant has hired is forgetful and clumsy. He often tosses food items onto the plate as it exits the kitchen, straining the waiters and pushing them to their limit.
Most days, things go about typically, providing pantomime entertainment for guests. One day, forgetting to put garnish, the chef runs over and tosses one olive, “with just the right energy,” on the pasta plate, and the entire contraption of plates comes crashing down. In many ways, the restaurant is living the idea of fission if we consider the chef as a source of neutrons – olive or other garnish, and the waiters as the unstable fissile isotope. The unstable isotope absorbs a neutron, which pushes it beyond its stability threshold, causing it to fission (split).
During fission, U-235 splits into two new elements and, in the process, releases energy and more neutrons. These sneaky neutrons can move about and find other unsuspecting U-235 nuclei and cause them to fission, releasing more energy and neutrons. This chain of events is essentially what’s going on in a nuclear reactor and how energy is released. It is like one waiter, when he falls, sends flying food and plate that destabilizes the next waiter, who then falls and destabilizes the next waiter, and soon, all the waiters are on the floor, creating a lot of noise, and all the food is ruined, leaving the customers awestruck and their stomachs grumbly.
Other conditions are essential for fission to take place in a nuclear reactor (light water reactor). First, the olive cannot be thrown at the waiter at a fast speed. It has to be gently tossed onto a plate. i.e. the neutrons have to be slowed down. You cannot be playing baseball with the olive. You are playing beer pong. In a light-water reactor (most nuclear reactors today), neutrons are slowed down by water. And second, enough waiters must be present within range of an olive to ensure that if the olive misses one waiter, it can find another waiter and doesn’t fall on the floor (this is why natural uranium must be enriched in U-235 before use in a light-water reactor).
Fission is a process that deals with atomic forces and results in a tremendous release of energy. The fission of U235 can release 200MeV of energy per fission. To give an idea of how large that is, 1kg of natural uranium, when enriched for use in a light-water reactor, can generate 45,000 KWh of electricity. The same amount requires 14,000 kg of coal or nearly 10,000 kg of gasoline. [10]
[1] Canadian Nuclear Association (2015, March 31). Uranium Mining in Canada. https://cna.ca/2015/03/31/uranium-mining-in-canada/
[2] Abate, T. (2017, February 17). How to extract uranium from seawater for nuclear power. https://engineering.stanford.edu/magazine/article/how-extract-uranium-seawater-nuclear-power
[3] Cohen, B. L. (1985). The Myth of Plutonium Toxicity. Nuclear Energy, 355-365. https://doi.org/10.1007/978-1-4684-4589-3_23
[4] World Nuclear Association (2021, April). Plutonium. https://world-nuclear.org/information-library/nuclear-fuel-cycle/fuel-recycling/plutonium.aspx
[5] Ojovan, M. I., Lee, W. E., & Kalmykov, S. N. (2019). An Introduction to Nuclear Waste Immobilisation (3rd ed.), pp. 433-461. Elsevier. https://doi.org/10.1016/B978-0-08-102702-8.00023-6
[6] Hedin, A. (1997). Spent nuclear fuel - how dangerous is it? A report from the project ‘Description of risk.’ pp. 10,16,17. (SKB-TR--97-13). Sweden
[7] International Atomic Energy Agency (n.d.). Getting to the Core of THE NUCLEAR FUEL CYCLE From the mining of uranium to the disposal of nuclear waste. Retrieved July 12, 2023, from https://www.iaea.org/sites/default/files/18/10/nuclearfuelcycle.pdf
[8] Cohen, B. L. (1990). The Nuclear Energy Option: An Alternative for the 90s (p. 176). Springer.
[9] World Nuclear Association (2021, April). Nuclear Fuel Cycle Overview. https://www.world-nuclear.org/information-library/nuclear-fuel-cycle/introduction/nuclear-fuel-cycle-overview.aspx
[10] The European Nuclear Society (n.d.). Fuel comparison. Retrieved July 12, 2023, from https://www.euronuclear.org/glossary/fuel-comparison/