Radioactive waste is a problem for us and the environment
is radioactivity dangerous? how long does it last?
This reading is part of a series: Nuclear Waste Disposal
Previously, I wrote about how nuclear fuel takes a beating in a nuclear reactor and how fission physically and chemically transforms the fuel, which gains radioactivity and becomes hot in the process. The transmutation of nuclear fuel creates fission products and actinides, new nuclei that are the source of this radioactivity. Generating energy from uranium makes waste a million times more radioactive than natural uranium.
Relevant from the archive:
On fission (Link)
Why is nuclear waste from a reactor so radioactive? (Link)
How can radioactivity from spent fuel be harmful?
Types of ionizing radiation:
A general idea of what types of radiation there are can be instructive in understanding how harmful nuclear waste can be to us. Think of it in three tiers – low, moderate, and high.
Alpha radiation is considered low-energy. It has a minimal ability to penetrate and can be easily blocked by a sheet of paper, skin or a few inches of air [6]. This means we can hold an alpha-emitting object in our hands and experience no harmful effects. However, ingesting alpha-emitting material can be very damaging to internal organs.
Beta radiation is moderate in intensity. We cannot hold a beta-emitting object without some protection. Lead-lined gloves or a metal or plastic sheet are sufficient to stop it [7].
Gamma and neutron radiation are high-energy radiation. Gamma radiation requires several feet of concrete or a few inches of lead to block them [6].
Alpha, beta and gamma radiation can penetrate materials and cause cancer or death if the exposure is high, but they lack the ability to make anything radioactive. Neutrons, on the other hand, make other materials radioactive. Neutrons are incredibly energetic and require thick hydrogen-containing materials (concrete or water) to block them [6].
What radiation dose can cause cancer?
There is no deterministic answer to what dose can cause cancer from external irradiation. However, past observations, such as with data from atomic bomb survivors of Hiroshima and Nagasaki, have provided estimates that have helped form guidelines.
National and international regulatory bodies assume a 5% probability of contracting fatal cancer from a radiation dose of 100 rem [8].
Does this mean that from a smaller dose, say of 10 rem, the risk would be 0.5%. And from 1 rem, the risk would be 0.05%? This is what the linear no-threshold model postulates. This model estimates the health effects of radiation-induced cancer and extrapolates the impact of high-dose radiation into a very low-dose, where insufficient evidence exists of radiation’s risk to health.
We all receive low-level radiation from various natural and artificial sources, like an X-ray exam at a hospital. The average background radiation from natural sources in the US is around 0.3 rem [1]. Colorado received more cosmic radiation because of its high altitude and thinner air. This, along with the higher uranium content in the soil, means that natural background radiation in Colorado is higher than the national average, at around 0.4 rem [9]. In Florida, which is at sea level and where the soil is deficient in uranium, natural background radiation is lower than the national average [10]. Despite this, the incidence rate of all cancer in Colorado is 12% below the national average, while in Florida is slightly above [11].
For Leukemia, the most radiation-specific type of cancer [10], the incidence rate in Colorado is 10% below the national average and in Florida is 24% above the national average [11]. So exposure to radiation at these lower levels doesn’t tell us much about cancer risk. Other risks in our daily lives are much more likely to cause cancer or death.
Many in the scientific community don't believe the model should be continued as the primary scientific basis for radiation protection standards (see image below). The claim is that many scientific studies have shown the model is no longer valid [17].
No matter how useful it may be for fostering scientific debate and making guidelines, the dispute that the model engenders distorts how the public perceives the danger of low radiation exposure. This has a knock-on effect on how the general idea of “nuclear” and “atomic” is viewed. If even a tiny amount of radiation is believed to be harmful by the public, their acceptance of nuclear energy will never be easy. It makes nuclear an easy target. This is a central reason why, despite the clean energy it offers and the enormous contributions it could have made to prevent global warming, it was vehemently rejected by environmentalists in the 1980s and 1990s. Now, of course, it is rejected because it will presumably take too long to deploy in sufficient quantity to prevent climate change.
Whatever the argument against it, the model makes it nearly impossible to underestimate the risk of cancer from a low dose. Guidelines rely on scientific fundamentals. But where information is insufficient, ethical considerations prescribe an abundance of prudence until knowledge is made available.
The linear no-threshold model is endorsed by both Nuclear Regulatory Commission (NRC) and US Environmental Protection Agency (EPA) [17].
How long does it stay radioactive?
Radioactivity is a long-term concern. If its impact were only over the short term, it wouldn’t have been such a problem.
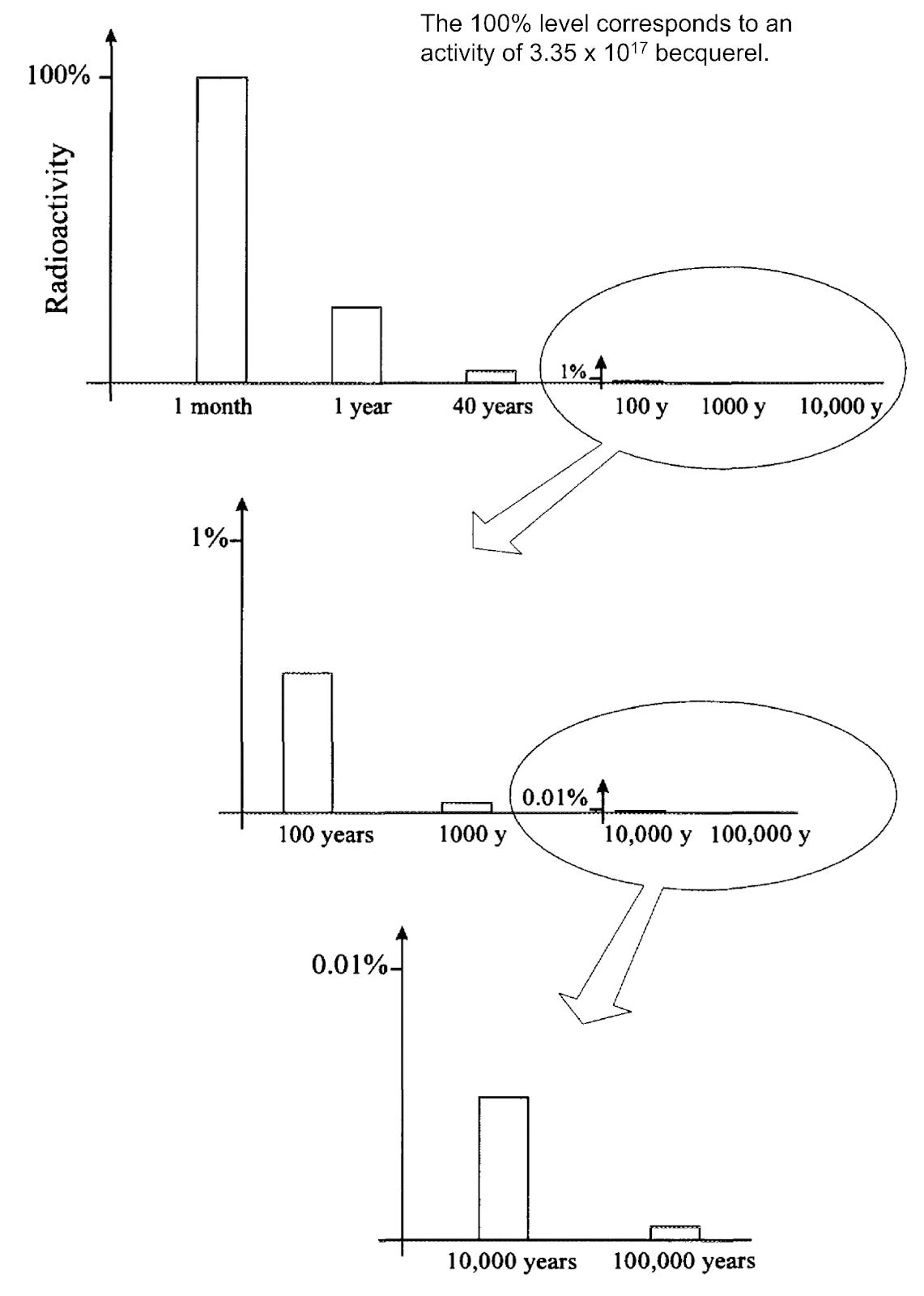
The first 100 years:
When the fuel exits the reactor, it is intensely radioactive. Fission products dominate radioactivity for the first 100 years [1].
Cesium-137 and Strontium-90 are the most radioactive part of the spent fuel during this early phase. They are both beta emitters, and Cs-137 also emits gamma rays. These two fission products are so radioactive that a small quantity, barely enough to fill the back of a pick-up truck, contains one-tenth the radiation that’s found in the core of a large nuclear reactor and generates enough heat to power more than 200 homes [2]. They have half-lives of about 30 years (radioactivity will fall to half in 30 years), but they stay highly radioactive for around 800 years before turning to low-level waste [3].
After 100 years:
In 100 years, the radioactivity of spent fuel falls to 1% of when it exited the reactor but is still at hazardous levels.
Short-lived isotopes Cs-137 and Sr-90 continue to be a problem but are slowly taken over by actinides (e.g. plutonium) in the radioactivity of spent fuel. Actinides dominate radioactivity after 500 years [1].
The actinide of importance after 100 years is americium-241, which has a half-life of 432 years. It is an alpha radiation emitter. It poses a significant risk if ingested, for example, from groundwater. It tends to stay in the body for a very long time, in bone, liver and muscle, and continues to expose the internal organs to radiation, increasing the risk of developing cancer [14].
As its radioactivity subsides, the plutonium isotopes Pu-240 and Pu-239 become the dominant radioactive source in the waste. Plutonium is also an alpha radiation emitter. It is most dangerous when inhaled. However, ingested plutonium from contaminated food or water is not considered a severe threat because the stomach does not absorb it easily [15].
After 10,000 years, when 0.01% of the original radioactivity is left in the spent fuel, the dominant source of radioactivity is Pu-239, which has a half-life of 24,000 years [1]. The proposal is to isolate the radioactive waste in a geological repository deep underground for as long as the waste is dangerous, which is at least 100,000 years.
It will take over 100,000 years for the radioactivity of the spent fuel to return to the level of uranium ore naturally occurring in nature.
How long is that?
The argument and concern that a geological repository’s integrity cannot be assured for 10,000 or 100,000 years with certainty; that, “you can be confident, but you cannot be absolutely sure that the repository will survive”; this argument and concern is not entirely out of place. We cannot be absolutely sure about anything that far into the future.
To give some idea of the time frame we are discussing here, humans started practicing agriculture only around 10,000 years ago [13]. Before that, we lived the hunter-gatherer lifestyle, but less sophisticated than the Flintstones. We used our hands and rock tools. Farming allowed us to establish permanent settlements, allowing civilizations to grow. Fast forward 10,000 years, and we are well on our way to becoming “one with the machines”. Our tools are highly complex and enable us to do things unimaginable back then. The world population of 5 million has now grown to 8 billion. Apart from the physical change that is obvious in our way of living and our technology, the software of our society has become highly complex. Economics, politics and institutions that define how we do things were nonexistent back then. Imagine what the world will be 10,000 or 100,000 years from today. We cannot be sure about the direction that our future will take. So how can we presume to have the ability to define the future of nuclear waste?
It is hard to argue against this point. The only counterpoint here is that – yes, the risk is there, that a repository would not survive that long. Still, we should weigh this risk against the benefits that nuclear power can provide and the harm to the environment that may be caused by using alternative and less dense sources of energy. I am not sure what the answer is. The way our demand for energy is growing, at some point, the only way to limit damage to the environment will be to use highly dense sources of energy. At some point, using an energy source that can deliver a certain MW of power from a plant occupying 50 acres of land will be preferable to a plant providing the same power by occupying 500 acres. One thing seems clear: if we want to strive towards progress, as a policy, we shouldn't shun any technology that can offer substantial benefits. Instead, we should try to find solutions to the few problems that, once solved, will make such technologies widely attractive. We cannot be certain that a geological repository won't fail, but we could potentially gain control over the way it fails.
References:
[1] Hedin, A. (1997). Spent nuclear fuel - how dangerous is it? A report from the project 'Description of risk.' pp14, 16, 19, 21, 23, 24, 30, 39. (SKB-TR--97-13). Sweden.
[2] Cornwall, W. (2015). Deep sleep. Science, 349(6244). p134. https://doi.org/10.1126/science.349.6244.132.
[3] National Research Council (2003). Improving the Scientific Basis for Managing DOE's Excess Nuclear Materials and Spent Nuclear Fuel. p53. Washington, DC: The National Academies Press. https://doi.org/10.17226/10684.
[4] MIT (2003). The Future of Nuclear Power: An Interdisciplinary MIT Study. pp58, 158. Massachusetts Institute of Technology.
[5] NRC (2019). Backgrounder on Radioactive Waste. US Nuclear Regulatory Commission. https://www.nrc.gov/reading-rm/doc-collections/fact-sheets/radwaste.html
[6] NRC (2020). Radiation Basics. US Nuclear Regulatory Commission. https://www.nrc.gov/about-nrc/radiation/health-effects/radiation-basics.html
[7] Corkhill, C., & Hyatt, N. (2018). Nuclear Waste Management. p10. IOP Publishing. https://doi.org/10.1088/978-0-7503-1638-5
[8] ICRP (1991). 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann. ICRP 21 (1-3).
[9] Hendrick, R. E., Fullerton, G. D., Borgstede, J. P., Dodd III, G. D., Hendee, W. R., & Larke, F. (2011). Radiology Dose-Risk Smartcard. https://www.ucdenver.edu/docs/librariesprovider206/default-document-library/radiology-dose-smartcard.pdf?Status=Temp&sfvrsn=8c2b05b9_2
[10] Cohen, B. L. (1990). The Nuclear Energy Option: An Alternative for the 90s. p52. Springer.
[11] National Cancer Institute. State Cancer Profiles (2016-2020). https://statecancerprofiles.cancer.gov/quick-profiles/index.php?tabSelected=2&statename=unitedstates
[12] MIT (2011). The Future of Nuclear Fuel Cycle: An Interdisciplinary MIT Study. pp60, 161. Massachusetts Institute of Technology.
[13] National Geographic Society (2022). The Development of Agriculture. National Geographic. https://education.nationalgeographic.org/resource/development-agriculture/
[14] EPA (2023). Radionuclide Basics: Americium-241. U.S. Environmental Protection Agency. https://www.epa.gov/radiation/radionuclide-basics-americium-241
[15] EPA (2023). Radionuclide Basics: Plutonium. U.S. Environmental Protection Agency. https://www.epa.gov/radiation/radionuclide-basics-plutonium
[16] NRC (2022). Doses in Our Daily Lives. US Nuclear Regulatory Commission. https://www.nrc.gov/about-nrc/radiation/around-us/doses-daily-lives.html
[17] NRC (2021). Nuclear Regulatory Commission: Linear No-Threshold Model and Standards for Protection Against Radiation, 86 vols. Federal Register. https://www.federalregister.gov/documents/2021/08/17/2021-17475/linear-no-threshold-model-and-standards-for-protection-against-radiation